
The hydrogenation of castor oil, monomer acids and dimer acids with Evonik’s MONCAT® catalysts
Within the oils and fats industries, the most discussed hydrogenations involve the reduction of typical triglycerides (TG), and the reduction of split free fatty acids (FFA) from these TG. However, within these industries lie other economically important specialty hydrogenations – where the selection of the right catalyst and the right reaction conditions are of the utmost importance, decisive for technical and financial success.
These specialty applications may have little in common at first glance, but what unites them – and differentiates them from other commercial catalytic hydrogenations on the market – is the key role the reaction temperature plays in both instances. In the case of castor oil, too high a temperature and the product is ruined; with dimer and monomer acids, too low a temperature and hydrogenation is not achieved.
Although there are similarities to the more renowned hydrogenations in the oils and fats industries – the typical TG and FFA reactions – there are also major differences; both of which are discussed below.
The hydrogenation of castor oil with MONCAT® 2021
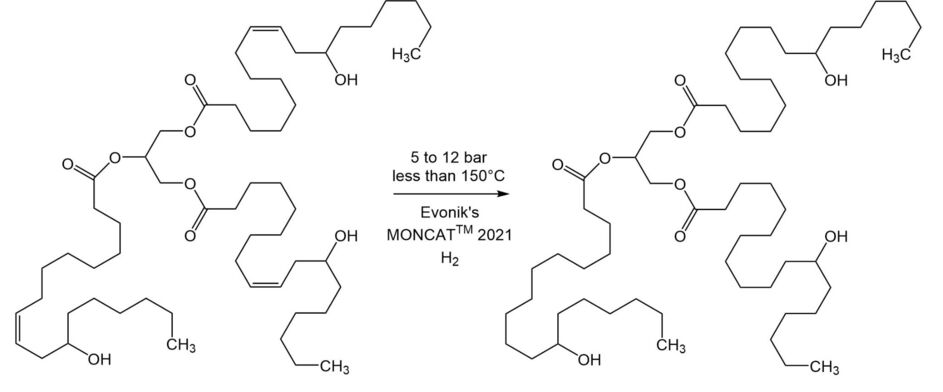
Castor oil is a very special TG, where more than 89% of the fatty acid chains are ricinoleic acid with a hydroxyl group on the 12th carbon[1]. These hydroxyl groups are polar in nature, which gives castor oil its unique properties – such as a higher density; compatibility with resins, polymers, and waxes; a viscosity that markedly withstands temperature changes; and it leaves very little residue when it burnsi.
Whereas typical TG hydrogenations are very robust processes with temperatures up to 200°C and pressures less than 5 bar, castor oil requires much milder temperatures and higher pressures that facilitate the reaction, while at the same time retaining its precious hydroxyl groups. Reaction temperatures higher than 150°C tend either to remove the hydroxyl groups altogether – converting it to just a standard fat or oil – or dehydrogenate them to the unwanted ketones.
The use of higher hydrogen pressures (around 5 to 12 bar) facilitates this reaction at temperatures lower than 150°C by increasing hydrogen availability for the rapid and selective conversion of the ricinoleic fatty acid chains to their 12-hydroxystearic acid (12-HSA) analogs.
Figure 1 displays the hydrogenation of castor oil where the preferred hydrogenation catalyst for this reaction is one that provides selective high activity at low temperatures with fast filtration; Evonik’s MONCAT® 2021 fulfills these needs and is the most widely used catalyst for this application.
Hydrogenated castor oil is normally saponified to preserve the hydroxy groups[2] and processed to mostly 12-HSA for use in cosmetics, pharmaceutical intermediates, wax mixtures, high-performance greases, polishes, inks, and hot melt adhesives[3].
Dimer and monomer acid hydrogenation with MONCAT® 1991
And on the other side of specialty applications, we look to hydrogenate unsaturated dimer and monomer acids made from processed split free fatty acids (FFA). These FFA can undergo a multitude of reactions over solid acid catalysts (generally montmorillonite) at 230 to 250°C and 5 to 10 bar to form a mixture of monomer, dimer, and some trimer acids, [4],[5],[6],[7] as seen in Figure 2.
These components are distilled, and the dimers can be hydrogenated to create nontoxic oleochemicals that improve the flexibility, elasticity, temperature stability and hydrophobicity of polymers[7]. They are also used in polyamide resin curing agents, alkyd resins and polyesters[7].
Additional applications using saturated dimer acids include oil field drilling rheology modifiers, lubricants, hot-melt adhesives, and epoxy curing agents iv. Isostearic acid makes up 35 to 55% of the hydrogenated monomer acids, and it is normally converted to derivatives used in engine lubricants and cosmetics iv.
It is known that nickel catalysts with smaller pores perform better than those with wider ones for FFA hydrogenation, due to the steric hindrance of nickel soap formation in these confined spaces[8]. On the other hand, the bulky structures of unsaturated dimer acids may need improved kinetics to be hydrogenated on very durable catalysts.
The hydrogenation of common FFA is usually performed at 200°C or lower to avoid unwanted side reactions and hydrogen pressures between 15 to 30 bars to inhibit nickel soap formation. Unlike for typical FFA, dimer acids can be hydrogenated at temperatures up to 240°C at 15 to 30 bars with MONCAT® 1991, due to its durability and ability to deliver very high yields under these conditions.
In other words, MONCAT® 1991 is not only an excellent free fatty acid hydrogenation catalyst, but it is also the preferred catalyst to produce saturated monomer and dimer acids.
![Figure 2. The formation and hydrogenation of monomer and dimer acids. [4],[5],[6],[7]](https://catalysts.evonik.com/en/products/brands/moncat/media/622362-desktop.jpg?rev=2b8bbf2d88a433184eb293cdf1b99b50)
Conclusion
The production of oleochemicals with special properties and sensitivities such as 12-HSA, monomer acids, and dimer acids require not only the right reaction conditions, but also catalysts with the right stability and activity at the required temperatures and pressures. Moreover, these catalysts also need excellent filtration properties for improved processing. MONCAT® catalysts from Evonik are the right choice for not only the typical fats and oils hydrogenation applications, but also the more demanding ones as those described here.
[1] H.B.W. Patterson, “Hydrogenation of Fats and Oils: Theory and Practice”, AOCS Press (2009).
[2]D.J. Anneken, S. Both, R. Christoph, G. Fieg, U. Steinberner, and A. Westfechtel, in Ullmann’s Encyclopedia of Industrial Chemistry, “Fatty Acids”, Wiley-VCH Verlaf GmbH & Co. KGaA, Weinheim (2012).
[3]12-Hydroxystearinsäure Wikipedia page, https://de.wikipedia.org/wiki/12-Hydroxystearinsäure#cite_ref-RömppOnline_5-0.
[4]Wu, L. Zeng, L. Viciu and T. Masuda, IHS Markit® Report, Natural Fatty Acids (June 2021)
[5]T.E. Breuer, in Kirk-Othmer Encyclopedia of Chemical Technology, “Dimer Acids”, John Wiley & Sons, Inc. (2000)
[6]D.A. Burg and R. Kleiman, “Preparation of Meadowfoam Dimer Acids and Dimer Esters and Their Use as Lubricants”, J. Am. Oil Chemists’ Soc., vol.68, No. 8 (1991).
[7]BCC Research Staff, BCC Research Report, Oleochemical Fatty Acids: Global Markets to 2023 (2019).
[8]C. M. Lok, Top Catal (2014) 57:1318-1324
