
The Use of Evonik’s Catalysts to Manufacture Saturated Free Fatty Acids for Oleochemical Applications
Fully saturated free fatty acids are an important feedstock for the oleochemical industry. On an industrial scale, they can be obtained from triglycerides by two principally different manufacturing methods:
- Splitting of triglycerides into glycerin and unsaturated free fatty acids, followed by the full hydrogenation of the free fatty acids.
or
- Full hydrogenation of unsaturated triglycerides, followed by their splitting into glycerin and saturated free fatty acids.
Both routes have their own unique challenges and advantages, and each case requires a different type of catalyst. Evonik offers the right MONCAT® catalysts for either route:
MONCAT® 1991 for the full hydrogenation of free fatty acids (obtained by triglyceride splitting)
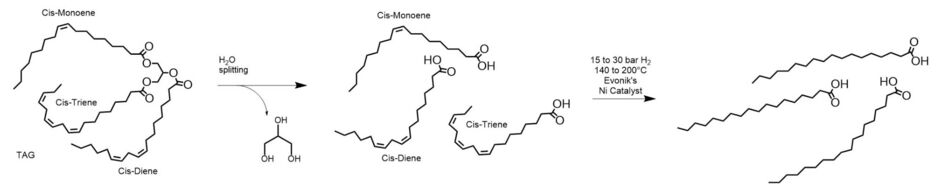
This route to fully hydrogenated free fatty acids is predominantly used by manufacturers in the Americas and Europe. The hydrogenation of free fatty acids is relatively fast and economical, and the initial removal of the triglyceride’s glycerin backbone increases the reactant’s concentration by 4-6% (dependent on the size of the fatty acid chains) in the reaction medium. However, as the free fatty acids undergo hydrogenation, they can also form unwanted nickel soaps:3,1
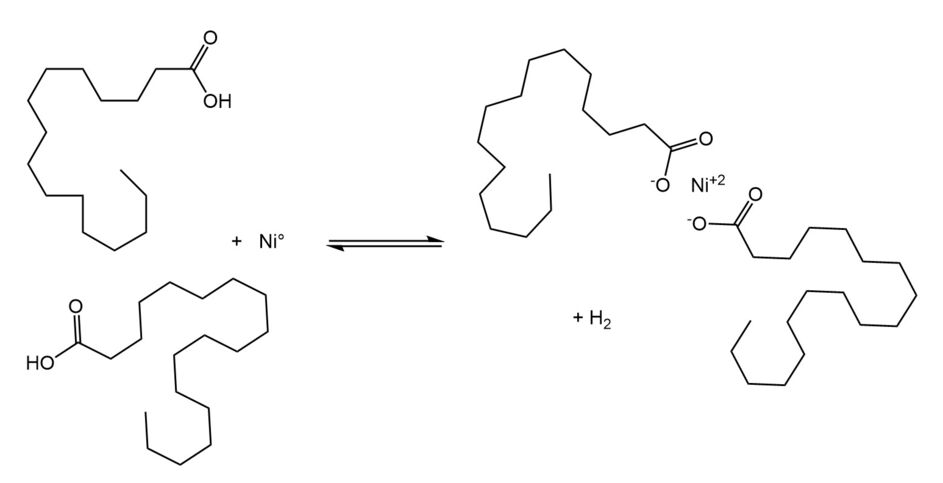
Nickel soap formation is an undesirable side reaction that leads to several unwanted consequences:
- it deactivates the catalyst via various mechanisms (e. g. chemical dissolution of nickel crystals as soaps, leading to a loss of active catalyst surface; nickel soap deposition, leading to blockage of active metal surfaces and/or blockage of active nickel sites in the pores, and others)
- it makes catalyst filtration after hydrogenation more difficult
- it increases the amount of solubilized nickel in the product, thus making post-hydrogenation purification more problematic
In short, nickel soap formation leads to higher overall process costs and must, therefore, be minimized as much as possible. The following measures can help to achieve this:
- Use a nickel catalyst designed to impede nickel soap formation3,1 (e.g., MONCAT® 1991)
- Use higher hydrogen pressures3,7 to shift the equilibrium in Figure 2 to the left
- Keep the water content in the reactant free fatty acids as low as possible3,1
- Use lower temperatures during the highest hydrogen demand in batch reactors for maintaining an adequate hydrogen concentration on the nickel surface. The reaction temperature can be increased after peak hydrogen demand to bring the reaction to completion
- Continuous hydrogenation processes (e. g. loop reactors) should moderate hydrogen usage to maintain its nickel surface population by adjusting the free fatty acid feeding rate and the reaction temperature
- Reduce the amount of time the catalyst is suspended in free fatty acids without the presence of hydrogen. Some hydrogenation systems suspend the nickel catalyst in free fatty acids prior to introducing it to the reactor and this should be done quickly to minimize nickel soaps. Catalyst filtration after the reaction is also in the absence of hydrogen and should be done expeditiously.
In essence, the hydrogenation of free fatty acids is a race between reaction completion and catalyst deactivation2, which is why it is critical to start with the best nickel catalyst for free fatty acid survivability, namely MONCAT® 1991.
MONCAT® 2021 for the full hydrogenation of triglycerides (followed by splitting them to glycerin and saturated free fatty acids)
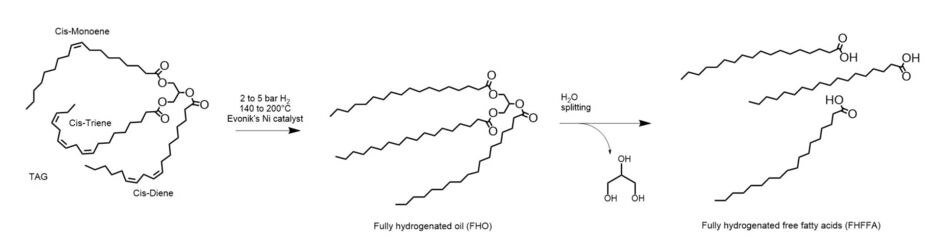
The second route to manufacture saturated free fatty acids is the full hydrogenation of triglycerides followed by splitting (see figure 3).
The inclusion of the triglyceride’s glycerin backbone increases the mass in the hydrogenation reactor from 4-6% for the same final product throughput. However, the reduced tendency for nickel soap formation allows for much lower hydrogen pressures (2 to 5 bars) which in turn lowers the investment costs for the required reactors. The oleochemical triglyceride feedstocks are not as pure as those for food purposes and small amounts of nickel soaps may form, however they can easily be removed by citric acid chelation after hydrogenation4. This is typically done by mixing the hydrogenated triglyceride with 0.01% to 0.05% weight of a 50% aqueous citric acid solution5 prior to filtration. Evonik’s catalyst best suitable for the full hydrogenation of triglycerides is MONCAT® 2021.
Evonik’s Oils and Fats Catalysts (OFC) along with their applications.
|
Product |
Ni wt.% |
Applications |
|
MONCAT® 1991 |
> 21 wt.% |
1. Full and partial hydrogenation of free fatty acids 2. Dimer and monomer acids |
|
MONCAT® 2021 |
> 21 wt.% |
Full and partial hydrogenation of triglycerides |
|
MONCAT® 5191 |
> 17 wt.% |
Sulfided catalyst for the partial hydrogenation and isomerization of free fatty acids |
|
MONCAT® 4181 |
> 17 wt.% |
Sulfided catalyst for the partial hydrogenation and isomerization of triglycerides |
Please contact your local representatives for more details.
Sources:
[1] D.J. Anneken, S. Both, R. Christoph, G. Fieg, U. Steinberner, and A. Westfechtel, in Ullmann’s Encyclopedia of Industrial Chemistry, “Fatty Acids”, Wiley-VCH Verlaf GmbH & Co. KGaA, Weinheim (2012).
[2] R.C. Hastert,” Hydrogenation of Fatty Acids”, J. Am. Oil Chemists’ Soc., Vol. 56 (November 1979)
[3] H.B.W. Patterson, “Hydrogenation of Fats and Oils: Theory and Practice”, AOCS Press (2009).
[4] R. D. O’Brien, “Fats and Oils: formulating and processing for applications”, 3rd ed., CRC Press, Taylor & Francis Group (2009).
[5] R. F. Ariaansz, “Hydrogenation in Practice”, AOCS Lipid Library internet page, https://lipidlibrary.aocs.org/edible-oil-processing/hydrogenation-in-practice.
